Contract Laboratory Services
CRO For pharmaceutical industries, large and small, we provide the resource for immune monitoring.
Challenges to monitoring T- or B cell-mediated antigen-specific immunity are ample. These start with the shipping and cryopreservation of live blood cells to the reliable detection of rare antigen-specific lymphocytes. We specialize in the logistic, separation, and cryopreservation of functional PBMC, establishing and performing such cellular assays in a regulated environment to support your studies, whether this be for preclinical or for clinical trials. We can assist you from the early planning stage to reporting auditable results.
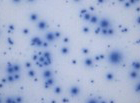
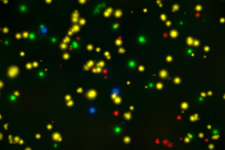
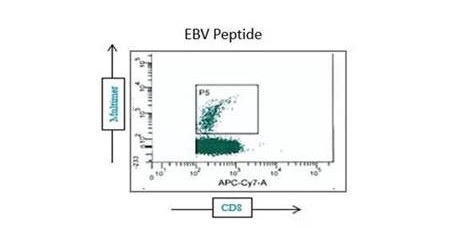
We are specialized in immune monitoring of CMI with emphasis on T and B cell (e.g. antigen specific plasma B cell and antibody producing memory B) evaluations within pre-clinical and clinical studies. Thanks to our in-house expertise and rigorous procedures, we can conduct all assays including ELISPOT, ELISA, NAb, NK-killer assay, CBA, FACS, proliferation, and more... in our regulated laboratory—whether to establish, to perform qualification-validation, test samples to satisfy FDA requirements or simply evaluate specimens for scientific significance.
We provide comprehensive solutions for PBMC sample processing: Serving as a central whole blood processing laboratory with optimized logistics, separation of PBMC, cryopreservation to ensure exceptional cell functionality of cryopreserved PBMC in the antigen specific ELISPOT assay and storage.
Our contract laboratory can test samples individually or in high-throughput format as needed and is capable of processing up to 450 samples per week.
We also provide high-throughput data acquisition, evaluation, and quality control, allowing us to process large assays within a relatively short amount of time. For our clients, we can schedule an initial telephone conference to discuss how our products and services can assist you in achieving your goals for a given project.
CTL has been providing contract laboratory services for government laboratories, major pharmaceutical companies, biotechnology companies, and academic institutions for over 15 years. Our company has been recognized by the Cancer Vaccine Consortium as its ELISPOT reference laboratory and has been awarded a contract by the U.S. National Institutes of Health (NIH) to validate the ELISPOT assay for pre-clinical and clinical trials. CTL is a member of the Society of Quality Assurance (SQA) and the Regulatory Affairs Professionals Society (RAPS). Our lab has been certified for Cell Viability by the International Society for Biological and Environmental Repositories (ISBER).
Our contract laboratory follows FDA guidelines for diverse regulations (GCP, GLP), GMP when applicable and is Part 11 compliant. In addition, we follow ICH guidelines, and our laboratory is CLIA/CLEP certified. We specialize in setting up custom Laboratory Developed Tests (LDT) for our clients in accordance with FDA Guidance for Bioanalytical Method Validation.
- California Clinical Laboratory License ID: COS 00800918
- Clinical Laboratory Improvement Amendments (CLIA) certificate ID : 36D1100176
- Maryland Department of Health, Office of Health Care Quality Medical Laboratory Permit
- New York State Department of Health Clinical Laboratory Permit
- State of Rhode Island and Providence Plantations Department of Health Clinical Laboratory License Number: LCO01323
- State of Pennsylvania Department of Health Clinical Laboratory Permit