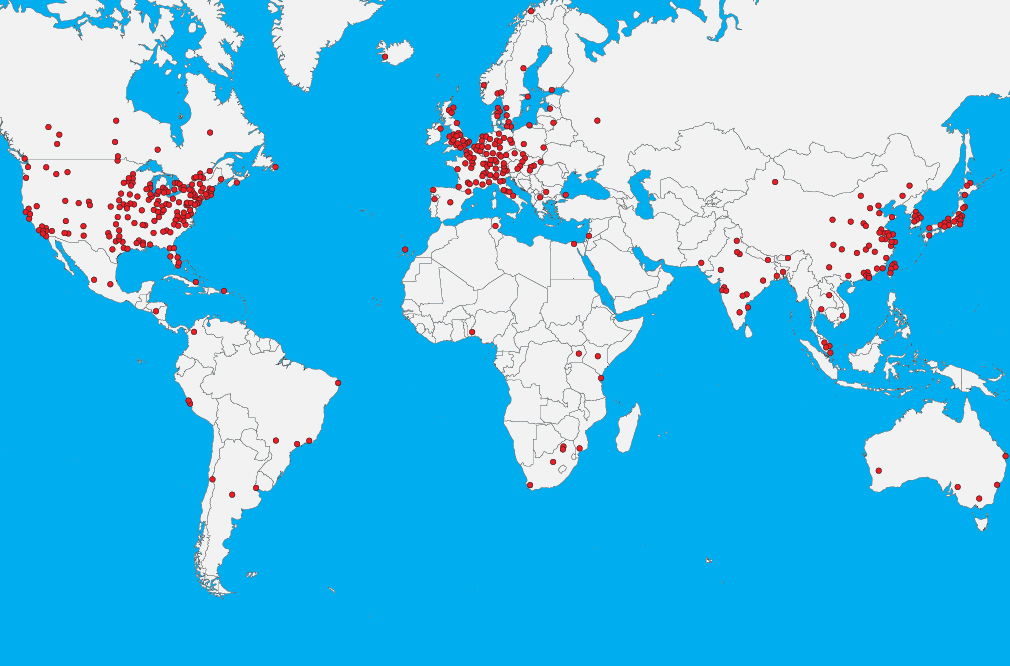
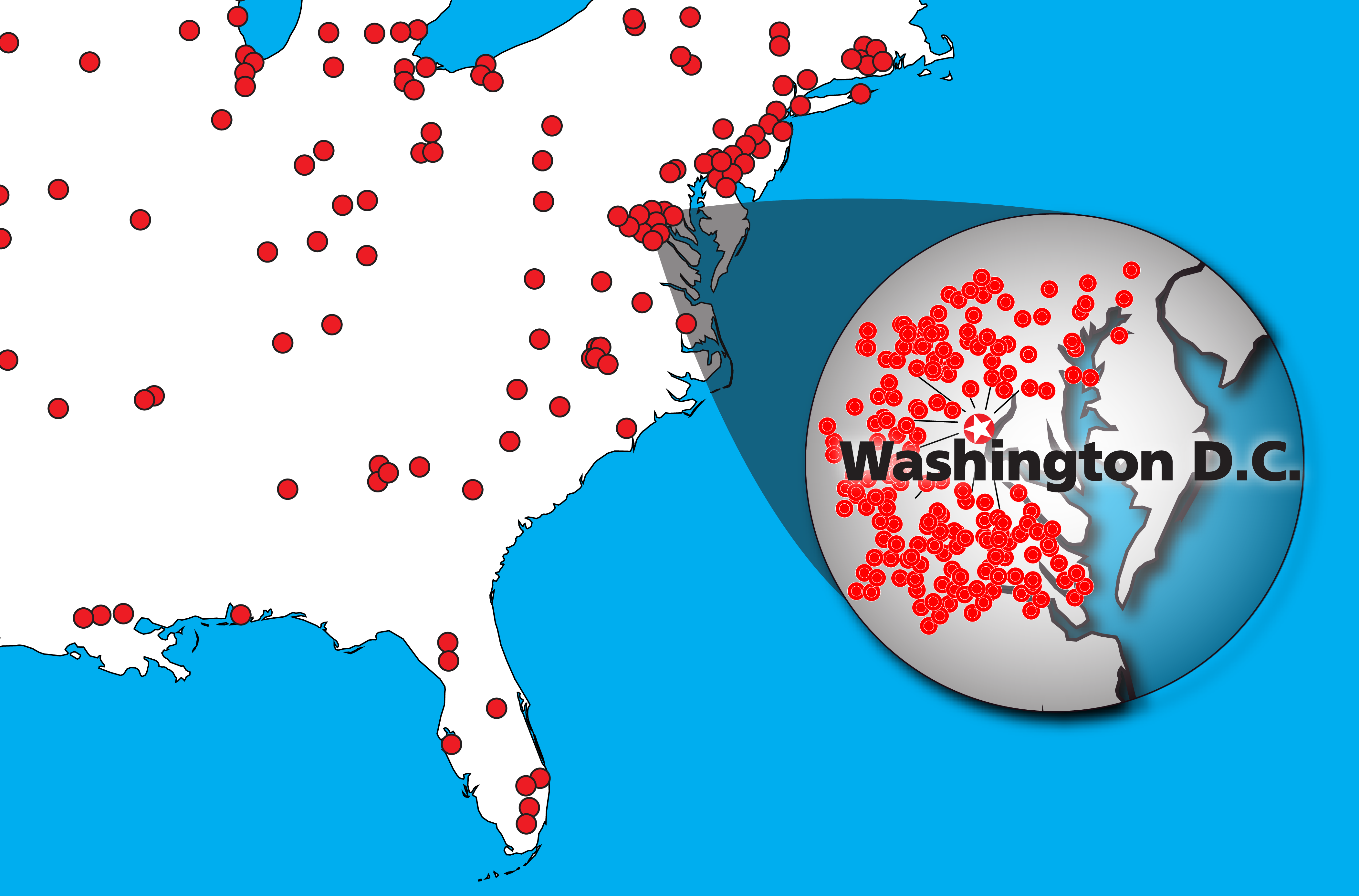
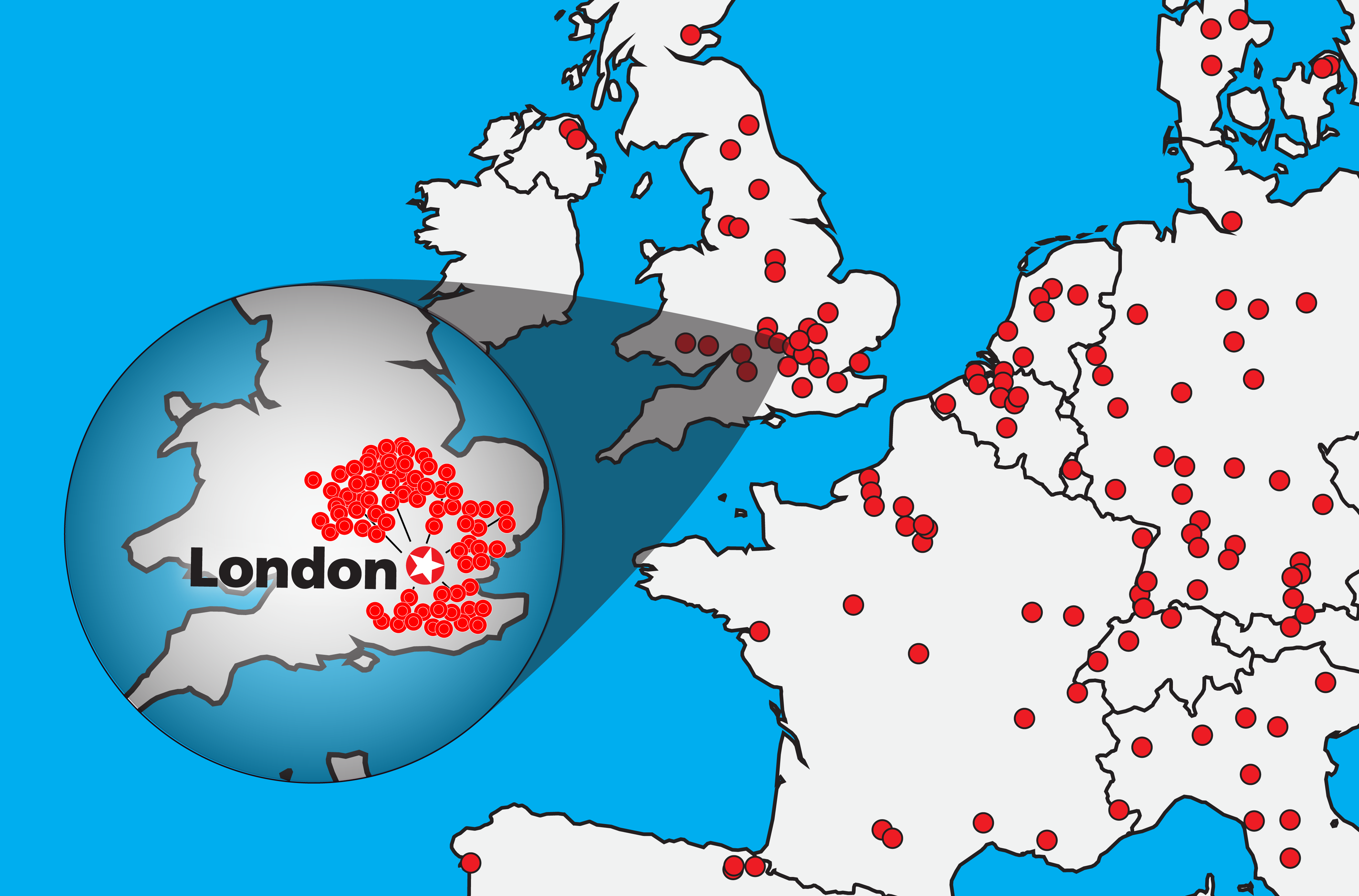
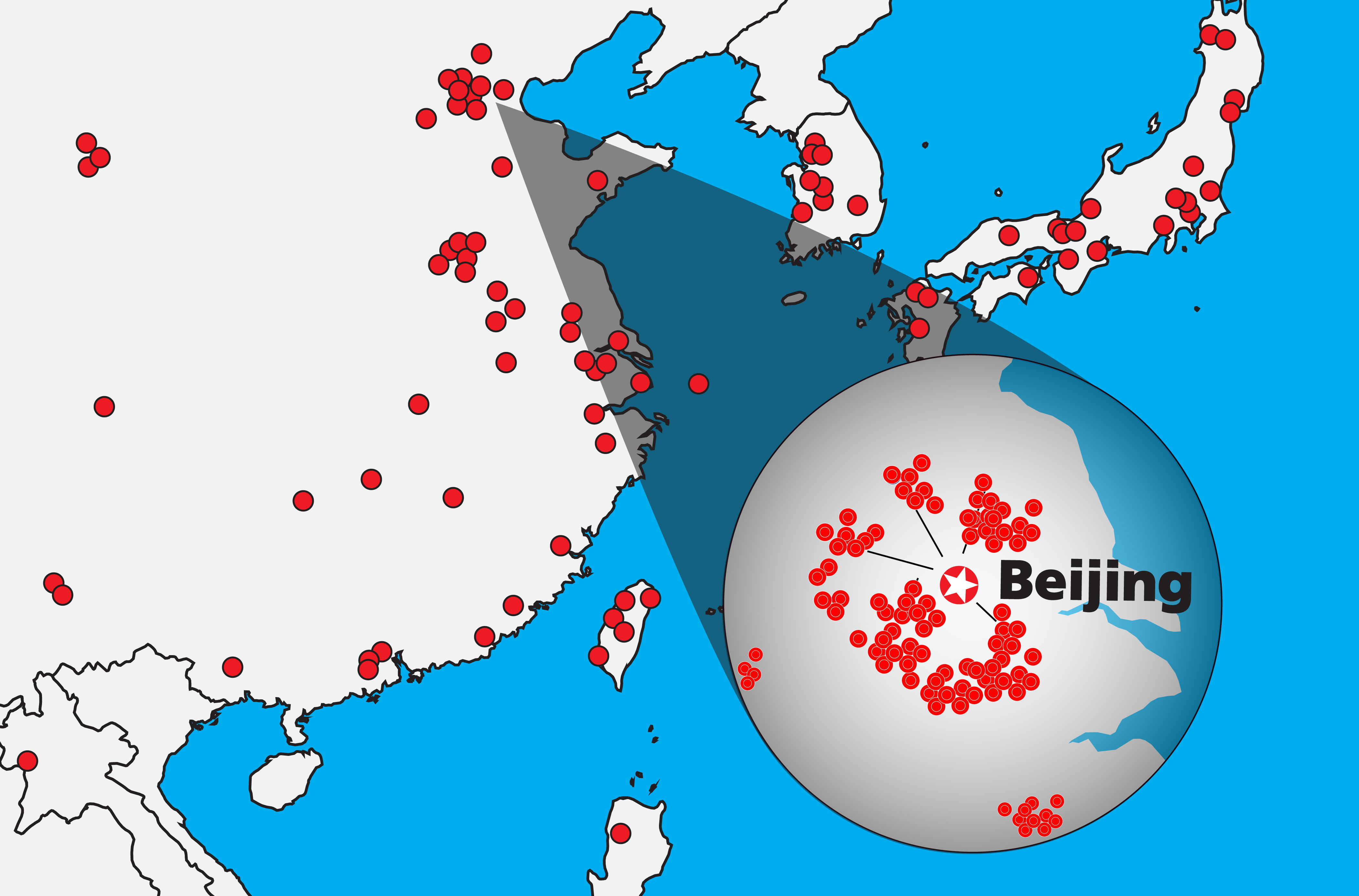
Leading ELISPOT
ImmunoSpot® stands for >25 years of leadership in immune monitoring by ELISPOT/FluoroSpot
CTL's founder and CEO, Prof. Dr. Paul V. Lehmann
Reliable detection of rare antigen-specific T and/or B cells has been a major challenge for immune monitoring. For over two decades, CTL has been leading this field with its ImmunoSpot® platform. View a lecture in which Prof. Dr. Paul V. Lehmann (see CV), CTL's Founder and CEO, explains how.
Prof. Lehmann and scientists working with him were the FIRST to:
- Build an ELISPOT reader (US Patent 6,410,252 B1; filed 12/95) – marking the beginning of the CTL ImmunoSpot® Analyzer line which introduced objectivity, reproducibility, and audit trails in ELISPOT/FluoroSpot analysis. Today, thousands of our readers serve the “who is who” of the academic and regulated market world-wide.
- Introduce PVDF plates for ELISPOT assays (Science, 1996, 271:1728 and US Patent 6,140,252; filed 12/95), which transformed the ELISPOT assay that previously had the reputation of “cold fusion” into the robust platform it is today. The PVDF membrane is now universally used for ELISPOT/FluoroSpot. Read more about this discovery (pages 227-29 in Critical Reviews in Immunology, 2020, 40: 225).
- Define which cytokines and spot sizes are T cell-derived (and thus relevant for T cell diagnostics) and which are “bystander” cytokine spots (Journal of Neuroimmunology, 2005, 170:105and Journal of Immunology, 2002,168:545), introducing the basics for using ELISPOT as a scientifically-validated T cell diagnostic tool.
- Use ELISPOT to understand different qualities of immunity induced by vaccination, delineating different T cell effector classes (Science, 1996, 271:1728).
- First to establish that CpG PAMS induce Th1 immunity (Journal of Experimental Medicine, 1997, 186:1623).
- Establish the scientific principles of ELISPOT analysis, such as how to interpret different spot sizes (Journal of Immunology, 2001, 167:1353), introducing the basics of automated gating strategies that ImmunoSpot® applies.
- Use ELISPOT for measuring T cell avidity for antigen (Journal of Experimental Medicine, 1998, 187:2055), introducing an important additional parameter of T cell immunity that ELISPOT assays can provide.
- Apply ELISPOT to comprehensive determinant mapping (Journal of Immunology, 2000, 165:1641), also introducing the high-throughput capabilities of the assay.
- Show that, when following the CTL protocols, PBMC can be cryopreserved without loss of function in ELISPOT assays (Journal of Immunological Methods 2003, 278:79), enabling the testing of clinical samples in specialized central laboratories and to generate cryopreserved PBMC libraries for reference samples and streamlined human research.
- Perform assays with a minute amount of cell material, such as isolates of mouse brain or blood (Journal of Immunology, 2001,166:4757 and Journal of Immunology, 2005, 174:4598), showing ELISPOT’s unique efficacy in cell utilization.
- Introduce double-color ELISPOT analysis with the ability to detect cytokine co-expressing / “polyfunctional” T cells with the same sensitivity as ICS (Journal of Immunology, 2000, 164:1862).
- Introduce granzyme B (Journal of Immunological Methods, 2000, 240:143) and perforin ELISPOT assays (AIDS Research and Human Retroviruses 2007, 24: 62) for detecting effector CD8 cells, and distinguishing them from memory cells that produce IFN-γ only, and TRAIL assays to detect helpless CD8 cells (AIDS Research and Human Retroviruses, 2008, 24:1175).
- Use ELISPOT to study transplant immunity (Transplantation 1997, 64:292, and US Patent 5,939,281; filed 9/96).
- Pioneer autoimmunity using ELISPOT (Journal of Experimental Medicine, 1996, 183:1561).
- Study adjuvant-guided T cell responses (Journal of Experimental Medicine, 1997, 186:1623).
- Use ELISPOT to study T cell immunity to HIV (Journal of Immunology, 2000, 164:3723-32).
- Use ELISPOT to study anti-tumor immunology (Journal of Immunology, 2003, 171:3941-6).
- First to establish that ImmunoSpot® is a robust T cell monitoring platform that generates highly reproducible results in different laboratories (Journal of Immunotoxicology, 2009, 6:227).
- First to introduce reference standards for regulated ELISPOT testing
- Introduce ELISPOT to diagnosing Multiple Sclerosis (Clinical Immunology, 2014, 152:20).
- First to harmonize ELISPOT between laboratories (Journal of Immunotoxicology, 2009, 6:227, and Cells, 2015, 4:21).
- To characterize Th1, Th2, Th17 immunity and polyfunctional T cells in HCMV infection (Viruses, 2015, 7:4417).
- Introduce and validate 384 well ELISPOT assays (Cells, 2015, 4:71).
- Study the impact of apoptotic cells within PBMC on ELISPOT results (Cells, 2015, 4:40-55).
- Systematically study the (lack of) benefit of PBMC resting (Cells 2012, 1:313).
- Systematically study interference of peptides within peptide pools (Viruses 2012, 4:2636).
- First to establish a CLIA certified, GLP-compliant (registered 6/09) laboratory.
- First to introduce rules for objective ELISPOT counting based on the statistical analysis of spot size distributions (Cells, 2015, 4:56).
- First to establish the rules for identifying positive ELISPOT test results (Cells, 2015, 4:96).
- First to introduce Reagent Tracker Dyes permitting to quality control for verifying plating accuracy in ELISPOT tests. (Cells, 2018, 7:3).
- First to establish that the detection of Th2 and Th17 cells by ELISPOT needs to account for their prolonged activation kinetics (Cells, 2017, 6:47).
- First to point out that experimentally verified CD8+ T cell determinants are not predictably immune dominant (Cancer Immunology, Immunotherapy, 2016, 65:847).
- First to experimentally work out the rules for multi-color FluoroSpot analysis establishing that simple center of mass algorithms are insufficient for identifying cytokine co-expressing T cells (Methods in Molecular Biology, 2018, 1808:95).
- First to perform 4 color T cell FluoroSpot for T cell lineage definition (Methods in Molecular Biology, 2018, 1808:51).
- First to perform 7 color B cell FluoroSpot to simultaneously detect all antibody classes and subclasses (Methods in Molecular Biology, 2018, 1808:85).
- To introduce imaging-based cytotoxicity assays that can be read on ImmunoSpot® Analyzers (Cells, 2018, 7:35).
- To draw attention to the unreliability of serum antibodies to detect immune memory to HCMV (Cells, 2018, 7:75).
- To demonstrate the feasibility of brute force epitope mapping involving the testing of 552 peptides on individual human donors with only 70 ml blood (Frontiers in Immunology, 2019, 10:655).
- To introduce a universal positive control for CD8+ T cell monitoring, CERI (Cells, 2021, 10:248).
- To introduce a positive control for CD4+ T cell monitoring, CPI (Cells, 2017, 6:47).
- To successfully use mega peptide pools for cancer immune monitoring (Cancers, 2021, 13:223).
- To draw attention to the discordance between the predicted vs. the actually recognized CD8+ T cell epitopes (Frontiers in Immunology, 2021, 11: 618428).
- To draw attention to highly variable (aleatory) nature of CD8+ T cell epitope dominance even in HLA-allele matched individuals (Frontiers in Immunology, 2021, 11: 618428, Crt. Rev. Immunol, 2020, 40: 225).
- To draw attention that there is no consistently immune dominant antigen within HCMV and EBV proteins (and possibly in other complex antigen systems) (Frontiers in Immunology, 2019, 10:655).
- To introduce negative control mega peptide pools. Frontiers in Immunology. 2021, 12: 982 Frontiers in Immunology. 2021, 12: 982
- To deconvolute specificity versus chance- and cognate cross-reactivity in the T cell response to SARS-CoV-2. Frontiers in Immunology. 2021, 12: 982
- Polyclonal B cell stimulator mimicking T cell help for monitoring antigen-specific memory B cells by ELISPOT/FluoroSpot. Cells, 2020,9:433
- Affinity tag coating to enable reliable detection of antigen-specific B cells in ImmunoSpot® assays. Cells, 2021,10:1843
- Quality control for successful and even pre-coating of ImmunoSpot® plates. Learn more.
- Introducing full exome characterized ePBMC. Learn more.
- Introducing accurate frequency measurements to antigen -specific B memory cell detection by ELISPOT/FluoroSpot. Learn more.
- To show that virus-specific memory B cells by ImmunoSpot® more reliably reveal past infections than serum antibody measurements do. Learn more.
- Enable the development of B cell ImmunoSpot® assays for any antigen of interest Learn more.
- Introduce protocols that enable to measure the accurate frequency of antigen-specific B cells secreting all four immunoglobulin classes (or IgG subclasses) with only 0.4 ml of blood? Learn more.
- Introduce protocols that enable the use of B cell ImmunoSpot® assays for assessing the affinity distribution of the antigen-specific memory B cell repertoire? Learn more.
- Introduce artificial intelligence for high content ImmunoSpot® analysis Learn more.
- Draw attention why standard serum antibody measurements cannot substitute for the direct assessment of memory B cells by ImmunoSpot® Learn more.
- To come up with guidelines and recommendations for validating B cell ImmunoSpot® assays Learn more.
- To demonstrate the amount of blood, or number of cryopreserved PBMC per vial, can be predefined for a planned B cell ImmunoSpot® test Learn more.
- To perform comprehensive characterization of antigen-specific memory B cells with limited blood volume, and in a high throughput compatible manner, as required for clinical immune monitoring.Learn more.
- To directly compare the specificity and technical requirements for performing flow cytometry- or ImmunoSpot-based detection of antigen-specific memory B cells.Learn more.
- To establish how to extend the detection limit of B cell ImmumoSpot® assays to permit detection of rare antigen-specific B cells.Learn more.
- To reveal that antibody feedback rather than the number of memory B cells determines the magnitude of a secondary response.Learn more.