Whole Blood Processing to Cryopreserved PBMC
High-Quality PBMC
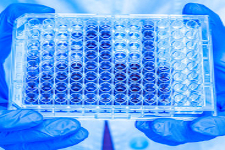
Our Contract Laboratory Service (CRO) team specializes in the logistics, separation, and cryopreservation of functional PBMC or subpopulations in our CLIA/CLEP-certified laboratory to support your preclinical studies in diverse species and your clinical trials (Phase I, II, and III).
Our CRO team has decades of experience processing PBMC for gene therapy, cancer, autoimmunity, vaccines and from various immunotherapy trials. Our proven cryopreservation method will ensure that your cells retain maximum viability and functionality, making our CRO an ideal Central Blood Processing Laboratory for your multi-center clinical trials.
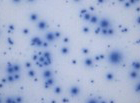
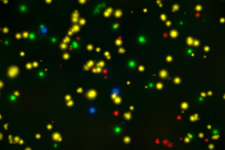
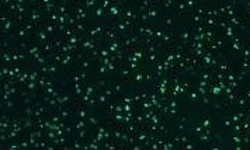
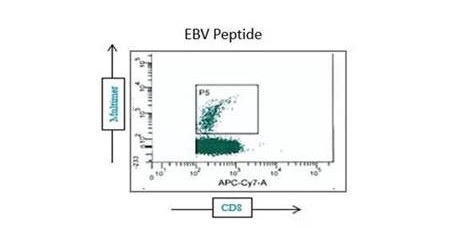
Our optimized cryopreservation method generates highly functional, viable PBMC for various immunoassays might this be for batched or high-throughput immune monitoring e.g. for antigen specific T and B cell ELISPOT as well as FACS assays.
In addition, we are processing and cryopreserving spleen cells, lymph nodes, cerebral spinal fluid, bone marrow and other tissues and fluids with highly maintained functionality.
Our CRO offers management and storage of cryopreserved vials e.g. PBMC and plasma/serum for further testing.
At our clients’ request, we provide whole blood shipping instructions, boxes, media, data loggers, and other items needed for shipping whole blood specimens from diverse clinical sites. The CRO also trains and qualifies laboratories involved in multicenter clinical trials for global studies.