T Cell ELISPOT Testing
Let Our Contract Research Laboratory (CRO) Team Fast-Track Your T Cell ELISPOT Needs
As pioneers in the ELISPOT arena, the CTL CRO Services team has more than a decade of proven expertise in the field of T cell ELISPOT.
Our CRO has developed and validated various customized T cell ELISPOT assays for pharmaceutical and biotechnology clients—large and small—spanning diverse antigenic systems, disease and treatment areas. We have successfully optimized and deployed high-throughput assays for clinical trials (Phase I, II, and III), testing for translational and preclinical studies.
Our CRO offers both, ready-to-go, kit-based T cell ELISPOT assay services and custom-designed assays to fit our clients’ specific project needs.
Let us help you to get your project on the right footing. Reach out to our experienced immune monitoring team today!
Our T cell ELISPOT assays can utilize either fresh or cryopreserved functional PBMC.
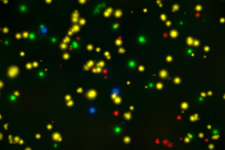
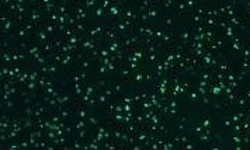
Optimized ELISPOT/FluoroSpot assays can reliably detect antigen-specific T cells even if they occur in frequencies of 0.0001% within PBMC (which is a single antigen specific analyte-secreting T cell within a million bystander cells).
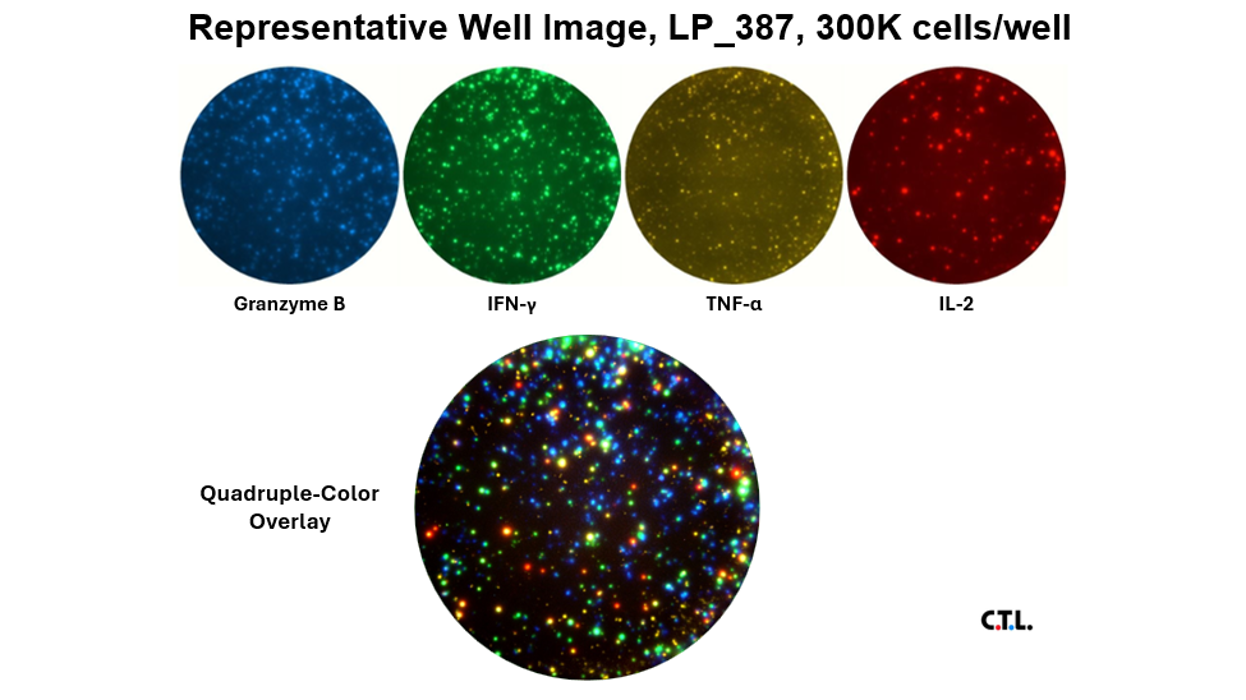
These immune assays range from single color/analyte ELISPOT assays to novel, high-throughput multiplexed FluoroSpot assays, that can be tailored to fit your specific project needs. Beyond clinical studies, our contract laboratory services have developed T cell ELISPOT assays for other species including non-human primates (NHP), rat, murine, feline, and porcine systems, just to name a few.
Our T cell ELISPOT/ImmunoSpot® method allows for complete characterization of immunogenicity:
- Testing T cell-mediated immunity for pre-clinical and clinical specimens
- High-throughput assay testing capabilities
- Epitope mapping with overlapping peptides
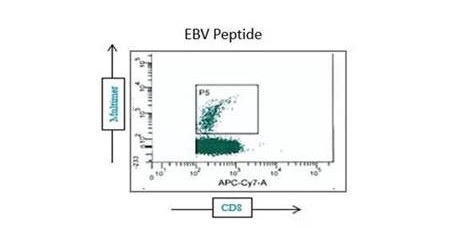
- Measurement of CD4+ and CD8+ T cell responses
- Performing multiple assays with the same sample material
- Immunotherapeutics (e.g. oncology, autoimmunity)
- Vaccine studies (e.g. infectious diseases, cancer vaccines)
- Transplantation (e.g. allo, MLR)
- Gene therapies (e.g. AAV-9)
- Safety assessments (e.g. ADA)
Based on the assay configuration, CD4+ and CD8+ subsets and associated effector and memory populations can be assessed.
Our highly trained immune monitoring experts can help coordinate multiple clinical sites and can walk clients through the complex aspects of T cell ELISPOT assay development, validation, deployment, and study logistics.