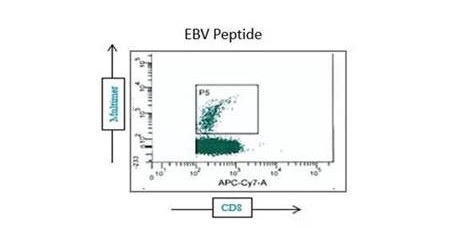
Neutralizing Antibody (NAb) assays
Accelerate Your Immune Monitoring Studies with our Contract Laboratory (CRO) Services
Neutralizing antibody (NAb) activity plays an important role for the immune system's response to viral infections, vaccines, and immuno-therapeutics and more. NAb assays are functional assays evaluating the reduction of viral infection in vitro in the presence of NAbs. These assays can be of particular importance in evaluating vaccines efficacy or gene therapy response in preclinical and clinical studies (phase I, II, and III).
NAbs are key indicators of host control of pathogens and immune responses generated by vaccines and immuno-therapeutics. As an example, our CRO immunoassay services include reliable readouts of NAbs specific to SARS-CoV-2 and various other pathogens and gene therapy vectors e.g. AAVs. Alternatively, NAb can bind to biotherapeutic drug products and prevent them from performing their intended biological function.
Monitoring such antibodies are of key importance in immunotherapeutic modalities that have the potential to induce such outcomes.
As pioneers in immune monitoring, our CRO team has proven expertise in performing NAb microneutralization assays. We offer customized assays for our pharmaceutical and biotech clients which we can develop, validate and perform the testing for our client specific studies.
Reach out to our experienced NAb immune assay team today!
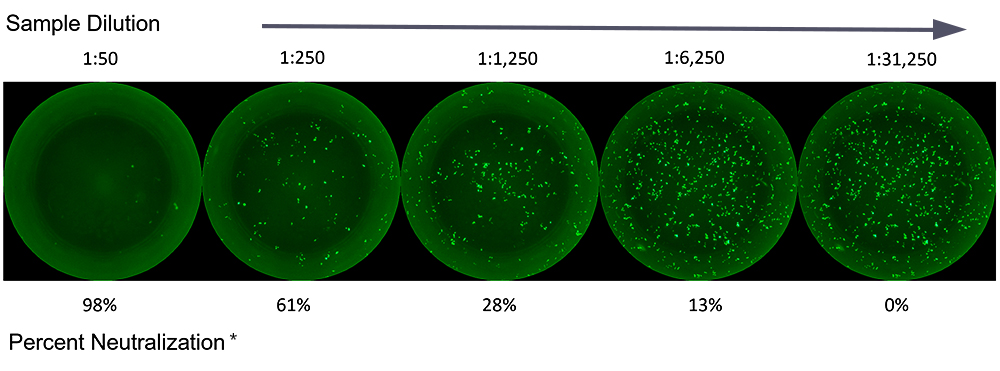
Neutralization Assays Provide High-Throughput Readouts
Our highly trained CRO NAb assay team utilizes microneutralization assays with relevant cell lines and labeled pseudoviruses and other antigens. Such assays are performed in our regulated biosafety level 2 (BSL2) laboratories using ImmunoSpot® analyzers and software.
For more than 20 years, some of the world's top pharmaceutical Clients have trusted us with their extensive immune monitoring needs.
Our experienced immune monitoring team can help you to reach your project milestones faster. For further information or a quote, please contact us today.