Whole Blood Processing to Cryopreserved PBMC
High-Quality PBMC for Clinical Trials
Our CRO team specializes in the logistics, separation, and cryopreservation of functional PBMC in CLIA-certified laboratories to support your preclinical studies and clinical trials (Phase I, II, and III).
Our staff has decades of experience processing fresh PBMC for clinical studies involving vaccines and immunotherapies. Our proven cryopreservation methods will ensure that your cells retain maximum viability and reliability, making our CRO an ideal Central Blood Processing site for your multi-center clinical trials.
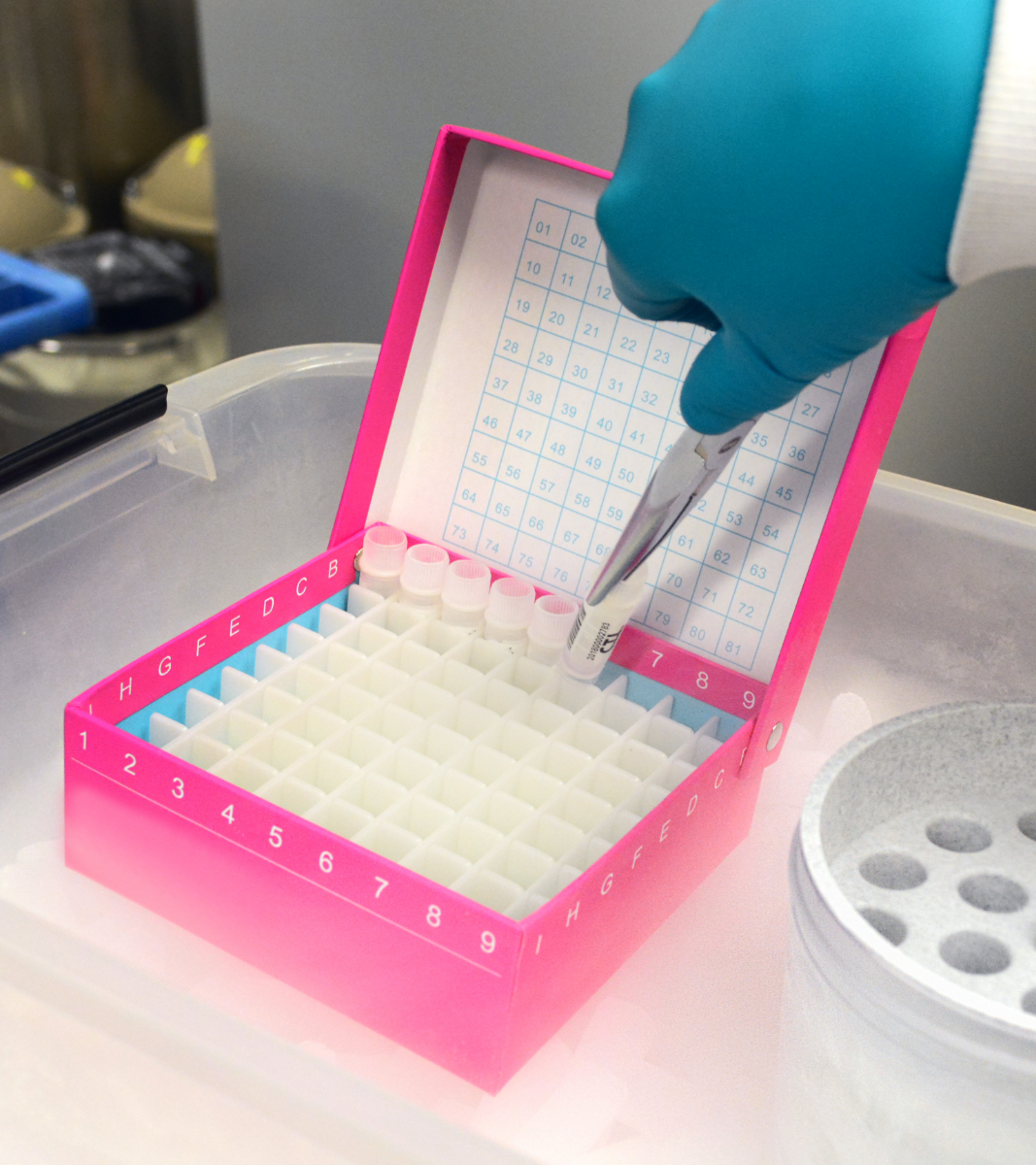
Isolate and Cryopreserve PBMC with CRO Services
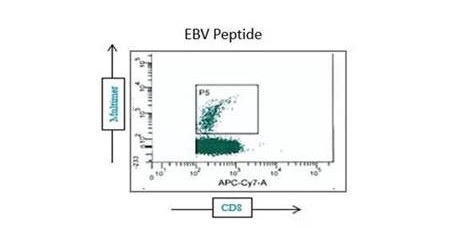
Our optimized cryopreservation method generates highly functional, viable PBMC that can be frozen and used in various immunoassays in batched, high-throughput immune monitoring schemes, including ELISPOT, ELISA, CBA/flow, and NAb assays.
Our CRO offers management and storage of PBMC and plasma/serum for additional testing.
Reach out to us to discuss your specific PBMC project needs. At our Client's request, we provide whole blood shipping instructions, boxes, media, data loggers, and other items needed for shipping whole blood specimens.
The CRO also trains and qualifies laboratories involved in multicenter clinical trials.